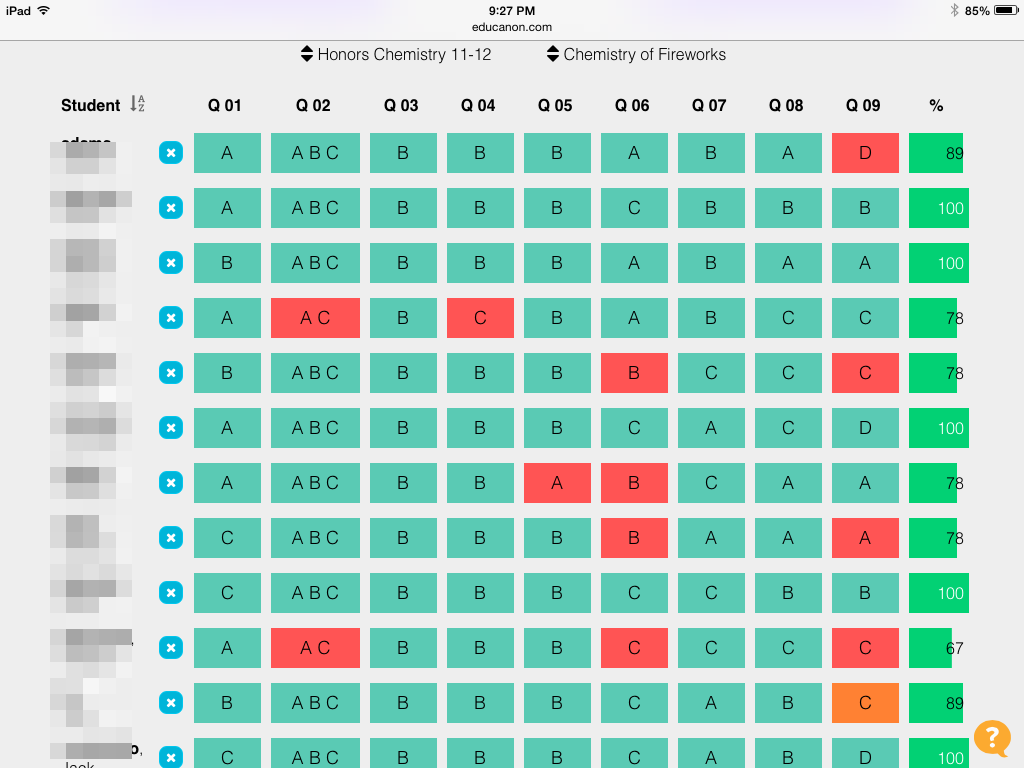
Here are some sample student responses:
These two are essentially the same, but I like them both for different reasons. The first with its solid demonstration of the model and the math, the second with its lovely conceptual simplicity.
This one is interesting. The right idea, but I am looking forward to talking about how half the volume - which this student clearly knows is a good answer - and this picture are not the same. Actually, a lot of students drew this same picture.
These two show the misconception that pressure and volume are the same. Or that gases don't really fill an entire container like we know they do. Good to know this misconception is still in the room.
Changing volume and number of particles were the most popular answers, but some kids addressed temperature nicely too.
Overall, the students did much better on this task. I liked the open-ended nature of this one. With at least three different answers, students had many opportunities to demonstrate mastery. I awarded full credit for any picture that would show an increase in pressure; I didn't hold them strictly to double the pressure since I was really checking for the conceptual relationship more than the mathematical one. The misconception uncovered here seems to be one that several students hold, so I hope it will be easy to dispel. In another week or so we tackle gas stoichiometry, so I am already thinking about the drawing question I put on that quiz to see if we make improvements over the limiting reactant question. Hopefully this confidence-building question will rub off on that one!
























.png)
.png)
.png)
.png)